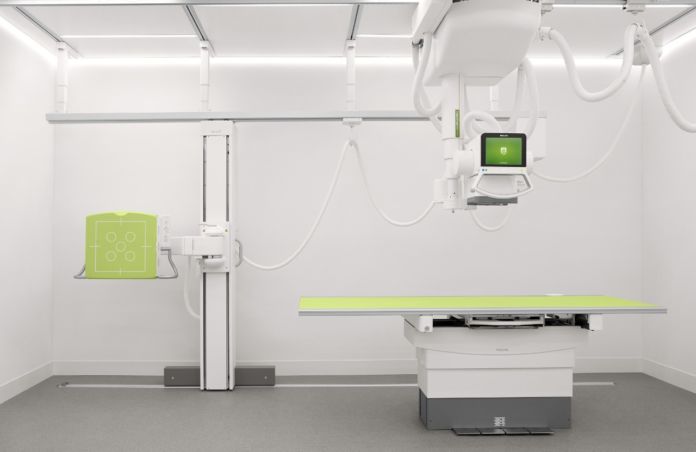
On Wednesday, Philips announced that it has received clearance from the U.S. Food and Drug Administration (FDA) to market its latest digital radiography system.
The DigitalDiagnost C90 can increase the number of patients who need to have x-rays done. It can also decrease the time required to come to a diagnosis. It offers healthcare organisations a flexible imaging solution that can be customised to help improve workflow and clinical outcomes, while also adding economic value.
This state-of-the-art radiography system provides a clear view of the part of the patient’s body that needs to be scanned while the patient is being positioned. This improves workflow as clinicians can be confident that the right area is being captured. A low X-ray dose exposure is also used.
With Philips’ UNIQUE 2 image processing and Bone Suppression software, radiologists can process clearer images. This ensures a more confident diagnosis with a lower chance of a costly and timely rescans.
In a press release, Daan van Manen says, “With solutions like the DigitalDiagnost C90, Philips is helping radiology departments get one step closer to achieving (a smooth and efficient patient-focused workflow).”
“This is being done through innovation that enables higher quality images while keeping the patient and staff experience at the forefront.” Van Manen is the Business Leader for Diagnostic X-ray at Philips.
Source: www.philips.com