The American Food and Drug Administration (FDA) has approved the PUREtrace labour registration system, made in Eindhoven, for the American market.
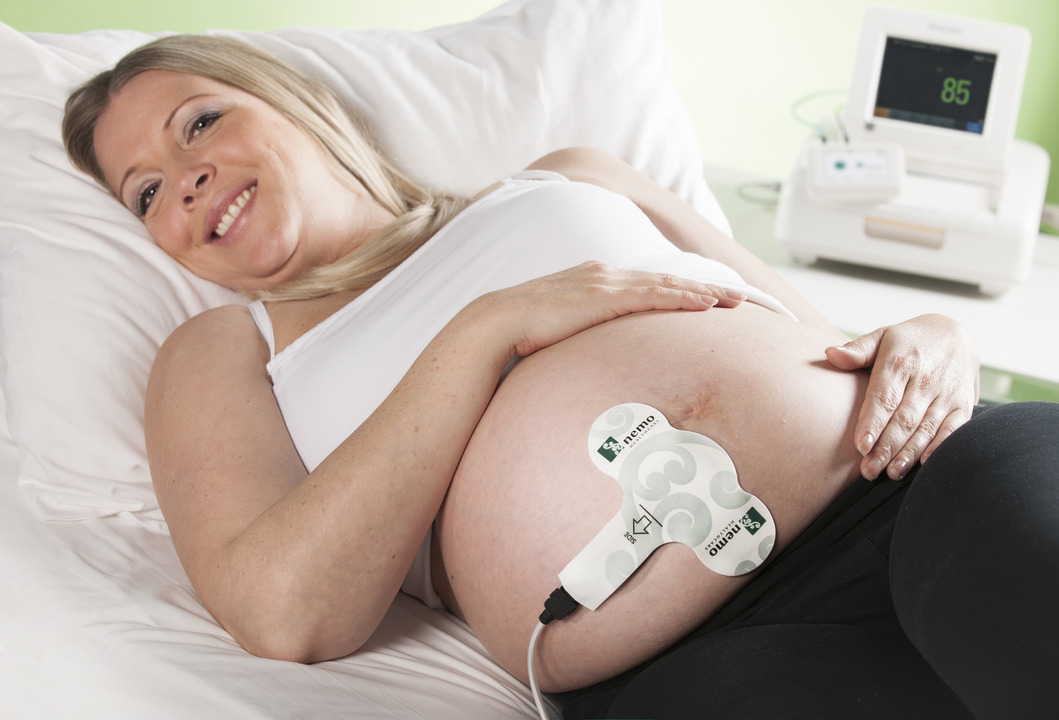
A safer and more comfortable method, the labour registration system uses a plaster placed on the abdomen of expecting mothers to measure labour activity before and during childbirth. The electrodes in the Graphium plaster measure contractions of the womb.
Labour activity is transmitted through the plaster to a monitoring screen. The PUREtrace module is available in several variants to enable coupling with a variety of monitors.
The product is now also available in the United States. Nemo Healthcare CEO Hans Brons said “The FDA’s approval is an important milestone for Nemo Healthcare. Our innovating and patient friendly technology is now also available to Healthcare professionals in the United States. With the ultimate goal of bringing foetal monitoring to a higher level and strive for better diagnostics and birth results.
Source: Studio040
Translator: Maurice